Why is COVID different than the flu?
As the current pandemic continues to unfold, people have compared COVID to other diseases to help them evaluate the risks of the disease and to understand why the world’s top experts reacted the way they did to COVID-19. One theme that frequently arises among proponents of a more lax COVID policy is that the mandatory shut-downs, mask-wearing, and banning of gatherings is a symptom of a media-driven overemphasis on the dangers of COVID. Even after the spike in COVID-19 deaths in New York early in the pandemic, I was still hearing people compare these COVID-19 deaths to the seasonal flu, arguing that the deaths attributed to the yearly seasonal flu were comparable to the COVID attributed deaths, and wondering why we shut down for COVID while we didn’t shut down for influenza. And some have pointed to the H1N1 pandemic in 2009, questioning why we didn’t have strong lockdown measures then, but we do for COVID. So let’s talk about it! Why has the worldwide medical community reacted so strongly to COVID, while there was a more muted response to the next most recent respiratory pandemic, H1N1?
First, we will need to talk about some basics- what is H1N1, why was it different than regular flu and what is coronavirus? Then we can start doing some comparisons.
Note: This post focuses on the initial COVID shutdowns back in March and April 2020. While COVID-related restrictions obviously continued after these months into the present, they are highly variable by location and require geographic-specific discussions as to the rationale. So this post is focused on the early “major” shutdowns, not the nuances of every state and city’s individual ongoing restrictions.
What is “the flu?”
First, a note about the word “flu” – people use the word “flu” to describe a lot of different diseases, including true influenza infections as well as the common cold and the “stomach flu”. However, when doctors use this word, they are generally referring to influenza viruses, which are a family of viruses that include both the seasonal flu and pandemic strains like H1N1. Influenza infections have the potential to be much worse than the common cold: the cold very rarely causes more than a stuffy nose and mild fever, while influenza more frequently causes considerable fatigue, body aches, fever, and chills. Influenza also has a nasty tendency to cause ‘post-viral pneumonia’, which is a much worse bacterial infection you get while your lungs are in a weakened state from having the influenza.
Influenza type viruses appear to have been with us since written history, if not longer, though it should be noted that before European colonization of North and South America, it appears that influenza was not endemic in Native American populations. Now the flu pushes through the entire world (except, maybe, for the Sentinelese) every year. Most years, we get a seasonal flu (though the specific strains change from year to year), but occasionally (typically about every 40 years), there is a pandemic flu. Why does the seasonal flu change every year? And what is it that makes a flu a pandemic flu?
How the seasonal flu changes every year
The reason we have to get flu vaccines every year for seasonal flu has primarily to do with the concept of antigen drift. An antigen is a part of the virus structure that the memory cells of your immune system can learn to recognize. For an explanation of how immune memory cells come to be and what role they play (also to look at cute gifs of puppies), I recommend
the blog post by Dr. Caitlin Miller on this very site. The flu virus has multiple antigens on it, but two of them are really important- the H antigen and the N antigen.
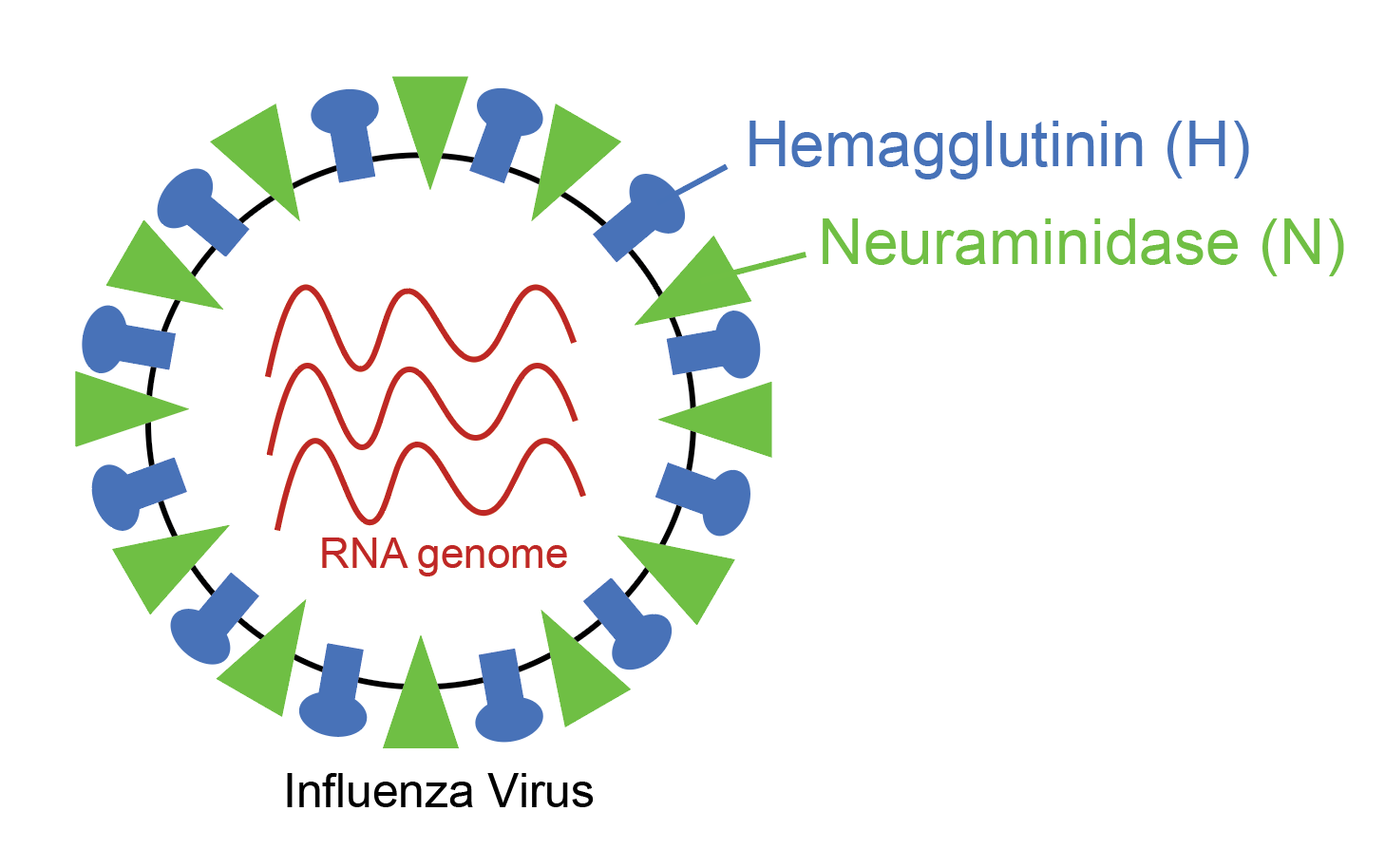
Flu viruses are named after what subtypes of H and N antigens they have on their surface, which will come into play later in this article. The problem is that influenza is a highly mutable virus, meaning that every time a new virus copy is made, pieces of the virus change just a little bit, so the antigens typically look a little bit different when we check up on them every few months. To make a comparison, we can imagine that the flu antigen is like a key, and the immune cell that needs to recognize the flu antigen is like your house lock. Well, the house lock and the key go together and when you put the key in the lock you are able to access your house. Now imagine that you filed down one of the ridges on your key and tried to put it in your lock. What would happen? Sure, the key would slide in, but because the pins don’t fit in the right place, you wouldn’t be able to turn the lock and access your house. That’s basically what happens with antigenic drift with the seasonal flu.
As the influenza virus goes around the world every year, the antigens change just enough so that when it comes back to the United States, our memory immune cells (which are very specifically made) do not recognize it very well. When our immune cells don’t recognize a virus, they aren’t able to mount a very fast immune response, so we get sick while our body figures out how to fight the virus. So how have we solved this problem? We depend on epidemiologists to figure out what the dominant strains of influenza are every year (usually by looking at flu strains in Australia), and we make a new annual flu vaccine that covers those specific strains. If you have ever been curious why your doctor tells you to get a flu vaccine every year, but other viruses you only need the shot when you’re a kid – this is why: viruses like measles have a stable genetic structure that doesn’t change much over time. This means that unlike influenza, we don’t have to worry about significant antigenic drift for measles, and a vaccine given early in life provides good immunity that lasts a lifetime.
Pandemic Influenza: Practically a Whole New Virus
But what about flu pandemics? How do those work? Pandemics are thought to work on the principle of antigen shift or reassortment/recombination. Essentially, the flu strain that becomes dominant has antigens that are so radically different from previous strains that the pandemic strain looks like a totally new virus to our immune systems. These novel strains can emerge by flu viruses mixing components, or when one of the animal influenza viruses gaining the ability to infect humans. Imagine the same key analogy, but your memory cells are all pin tumbler locks, like pictured above, and you’re instead presented with a barrel key. You would need to manufacture completely new locks to be able to recognize that key.

As it turns out, the different key type analogy can take us a bit further in understanding why flu pandemics have certain characteristics. If you recall the H1N1 flu pandemic in 2009 (also known as the Swine flu pandemic), you might remember that the elderly were less likely to die from the flu than usual, and younger people (especially children and teenagers) were unusually susceptible to death and morbidity from that particular flu. The reason was that the elderly (mostly those 60 and older) had already been exposed to a similar flu in their younger years, and their immune memory cells already had some idea what they were doing. Basically, they already happened to have barrel locks lying around, so the key that they were presented with was somewhat familiar. The elderly were hit with the standard antigen drift, while everyone else had to deal with antigen shift.
The H1N1 pandemic: what was different?
The Centers for Disease Control (CDC) has a timeline that gives a pretty good idea of how the response to the flu pandemic was carried out. Importantly, this was the first pandemic since the foundation of the World Health Organization (WHO) and the CDC, this was the first public health emergency of international concern that had ever been declared by the WHO and CDC, and the proper response to an international problem like this had only been theorized to that point.
Timeline of the H1N1 Pandemic
In early April of 2009, the first case of novel human H1N1 flu was identified. Soon after, community spread was confirmed in multiple states and was reported to the WHO. Cooperative work started immediately on sequencing the virus strain and on developing a vaccine. By the end of April, the US government declared a public health disaster of international concern and started releasing stockpiles of anti-influenza drugs; the CDC also published guidelines of how to deal with laboratory-confirmed infections in schools. Soon after this (early May), multiple schools were shut down to try to mitigate further community spread of novel H1N1 flu. There was a brief downtrend in the number of flu cases by mid-July, and clinical trial started on the vaccine candidates- almost 4 months after vaccine work started. Schools started again by the end of August and beginning of September, followed by a second wave of H1N1 flu. School closures continued to happen all over the United States in response to laboratory confirmed diseases. The first H1N1 flu vaccine doses were distributed in early October. The peak of the second wave of H1N1 flu occurred at the end of October. By the time there was enough vaccine for everyone, in late December, the virus had already somewhat died down, though it persisted in the community for several more months. The pandemic was officially declared over in August of 2010.
So, to recap, the interventions that were obvious were:
- Cooperation between the reporting country and the international public health community
- Early vaccine development based on an already well-established infrastructure for developing influenza vaccines
- Drugs that were known to be effective against influenza were released and used
- Schools and facilities and summer camps that had cases or outbreaks were closed to prevent further spread.
The things that are a little less obvious are some of the characteristics of the virus:
- The elderly were unusually immune to the virus.
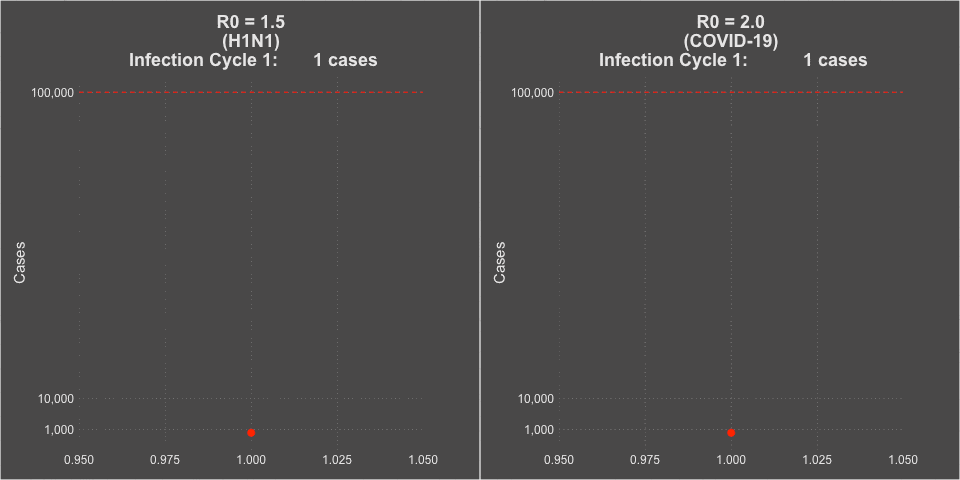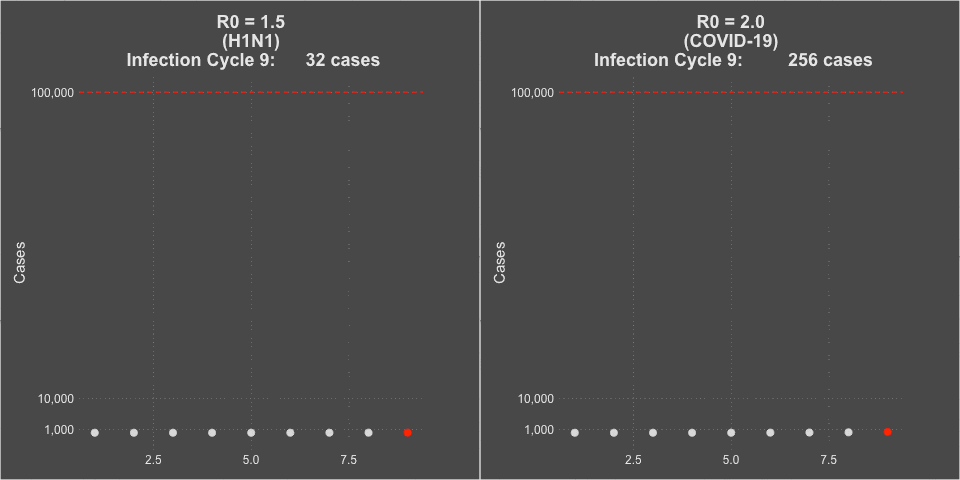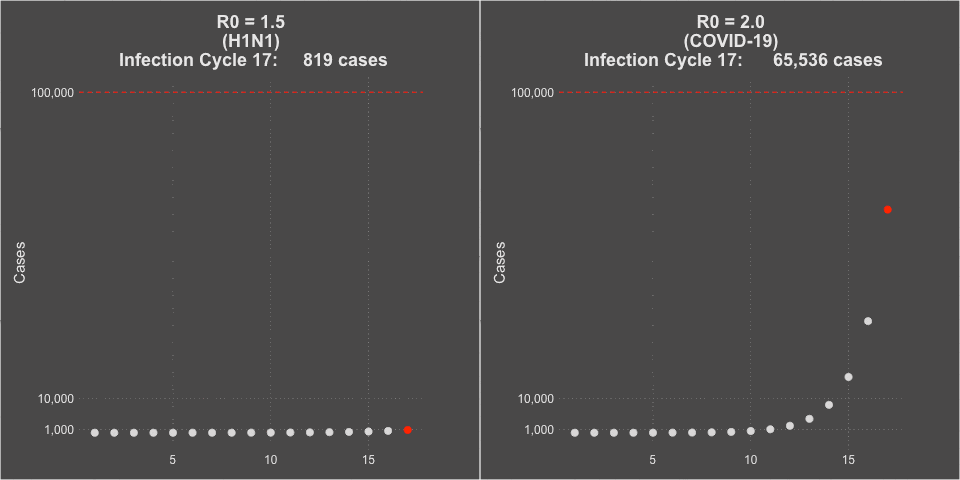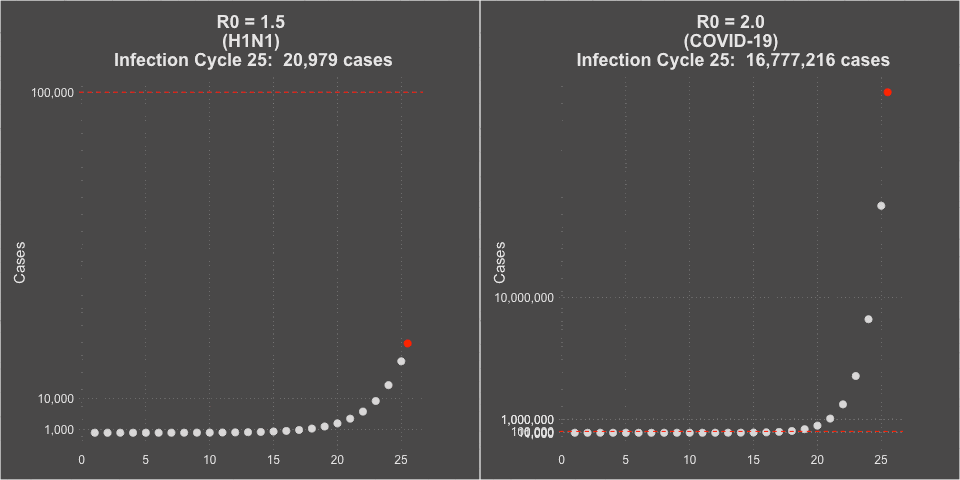
- The transmissibility of the virus (the R0 or Rt of the virus) was estimated at 1.5 at the beginning of the pandemic, but decreased to about 1.2 during the summer school vacation months with natural social distancing.
- People who got H1N1 had similar symptoms to people infected with seasonal flu, and had typical flu complications – the most common causes of death were respiratory failure from primary H1N1 infection, and post-influenza pneumonia.
- Available data at the time showed that masks did not appreciably decrease transmissibility of pandemic influenza.
- The estimate of case fatality rate in the United States was ~0.048%, or 48 deaths per 100,000 cases.
In the first year of the pandemic, 12,469 people were estimated to have died from H1N1 influenza in the United States. That’s about a thousand people per month. 80% of global deaths were younger than age 65.
SARS-CoV-2: The COVID-19 Pandemic
Now let’s talk about the thing on everybody’s mind and newsfeed.
Importantly, this is still an area of active research. We have had more than 10 years to study the mechanisms and transmissibility of H1N1 influenza, so we have significant retrospective bias. If may feel like it’s been forever, but remember that at the time of this writing, COVID-19 has only been known to exist for 9 months, and has only been known to be in the United States for 8 months. Real hub-bub about COVID-19 didn’t start until about 6 months ago, as of this writing. So, with that said, let’s dive into it:
SARS-CoV-2 virology compared to the flu
SARS-CoV-2 is from an entirely different family of viruses, the coronaviridae. We are actually quite frequently exposed to different coronaviridae. For the most part, these viruses just cause cold symptoms, or our body fights it off without making a fuss at all. Coronavirdae have their genetic code written in RNA, like influenza virus, and also undergo antigenic drift and antigenic shift (also known as reassortment/recombination). COVID-19 appears to have undergone reassortment/recombination. As far as we can tell, COVID-19 arose from a bat coronavirus that recombined with a related coronavirus from an another animal and was then able to jump to humans.
What were the early factors in decision making for COVID-19 policy?
Again, let’s remember that this is an evolving story, and the data we have are constantly being collected, updated and revised to better approximate the truth. The website Think Global Health has an exhaustive timeline that encompasses global coronavirus status, and I will be using that for my summarization of the events that may have led to the current public health policy guidelines of widespread shutdowns of various strictness.
At the beginning of December 2019, a ‘pneumonia of unknown etiology’ emerges in Wuhan, a city of 11 million people, in the province of Hubei, in China. The WHO is informed of a string of infections at the beginning of January 2020. By the middle of January, viral transmission is found in the neighboring countries of Thailand and Japan, and community spread (rather than direct contraction by exposure to animal sources) is suspected. By the end of January, the first case of novel coronavirus is identified in the state of Washington. Around the same time, Wuhan and a lot of the Hubei province is put under strict quarantine by the Chinese government to reduce further spread in mainland China. There are 830 confirmed cases and 25 deaths total (3% mortality rate), all in China, by this point.
Following major airline suspension of flights to and from mainland China, the United States imposes a ban on entry of ‘immigrants and non-immigrants’ from China to the United States secondary to known community viral spread of the pneumonia of unknown etiology. The ban does not prevent citizens, non-citizen spouses, asylum seekers, or permanent residents from returning from China. The WHO declares a public health emergency of international significance on the same day as the U.S. travel ban. Evacuation of foreign nationals from China begins in early February 2020. Soon after, the public health agencies of the G7 countries agree to coordinate their responses to the COVID-19 outbreak. Tests distributed by the CDC were found to be defective in middle February. Iran and South Korea confirm that they have cases in their countries- in Iran, the two confirmed patients died of COVID-19, while in South Korea it was found that 20 cases were linked to a single COVID positive woman. Iran and South Korea simultaneously recognize more and more cases with South Korea noting doubling of case numbers within 24 hours- both Iran and South Korea begin to restrict travel between cities and within individual cities. Within days, Italy pops up with 16 confirmed cases and immediately closes public areas. By the end of February, Iran shuts down universities and public spaces in 14 major cities; multiple Eurasian, middle Eastern, Asian and European countries have sentinel cases (mostly travelers from countries that had already declared infections); states of emergency have been announced on the U.S. west coast, many countries have banned large public gatherings. By this point, by WHO accounts, mainland China is developing fewer cases per day than the rest of the world. More than 2800 people have died from COVID-19 out of more than 84,000 cases (3.4% case fatality rate).
By this point, Iran and Italy have emerged as secondary epicenters of COVID-19. The United States sees small, but steadily increasing caseloads, but is not nearly as bad off as Europe. What happens next particularly shapes the view of how big a deal this virus is.
At the beginning of March, Italy imposes a nation-wide lockdown – the Vatican also closes St. Peter’s square and the Basilica to all tourists. On March 11, after 120 countries have declared infections totaling more than 142,000 over the course of about 12 weeks, including more than 5300 fatalities (3.7%), the WHO declares that COVID-19 is a pandemic. Stories pour in from Italy and Iran describing physicians having to make life-and-death decisions in the hallways of the hospitals because there are not enough hospital beds or enough ventilators to give everyone the care they need. Italian physicians write stories warning the world of the seriousness of this infection and the coming storm, and begging for social distancing guidelines to prevent a similar tragedy in other countries. The case fatality rate in Italy is particularly high, averaging 7%. At the same time China reports no new COVID-19 cases for the first time in 4 months, after stringent lockdown. By the end of March, Italy is sustaining more than 600-900 daily deaths secondary to COVID-19.
As New York, California and Washington act as sentinel cases in the United States, the public health experts of the nation come together to make recommendations. They recommend social distancing, and also begin to recommend general lockdown to slow undetected community spread, especially to nursing homes and care facilities where the most vulnerable population stays. The rationale is twofold: rapid spread of the virus will result in overload of existing healthcare structures leading to excess mortality simply because of insufficient machines and resources and staff to care for the number of sick; and countries that were effective in lockdown and contact tracing have controlled their case loads. Modeling estimates of the mortality of COVID-19 and associated conditions runs in the 200,000 to 1 million persons range. The advisement to lockdown is taken differently by different groups- many citing concerns about the economic impact of hampering travel and consumption. Despite public health recommendations from the COVID-19 Task Force, other high-level political figures send mixed messages about the seriousness of the COVID-19 pandemic.
The rest of the story is important too, but the purpose of this section is to see what led the public health officials of the United States to recommend lockdown.
So, why was COVID different?
- There were concerns that there was not early enough reporting from China that there might be a novel emerging respiratory illness. It seems like China reported that there was something going on before the virus was known to have spread to other countries, but it was several weeks before the WHO was informed. Regardless of this, even when a virus is reported as early as possible, as with H1N1, the virus has already entered the community and is spreading.
- COVID-19 is far more infectious than influenza. The transmissibility of the virus (the R0 or Rt of the virus) was estimated at 2-3 without social intervention. In countries that instituted strong social distancing interventions and shutdowns, the Rt of the virus was driven down to less than 1, and curves would flatten and decline. You can see maps of the calculated Rt of the virus for each of the 50 states over time at this website– it even includes when lockdowns were implemented and removed. Places that had infection but subsequently did strict contact tracing and that population level commerce and social shut down showed improvements in COVID-19 case rates.
Model of H1N1 (R0 = 1.5) and COVID-19 spread (R0 = 2.0)
3. The Case Fatality Rate is far higher than H1N1. The case fatality of COVID-19 ranged between 1.2% to 10.8% in different countries. In the United States, the case fatality, as of this time, is 3.1%, or 3100 deaths per 100,000 confirmed cases. Remember the true mortality rate of an infection is difficult to calculate early in a pandemic (and improves over time as doctors learn how to treat the disease).
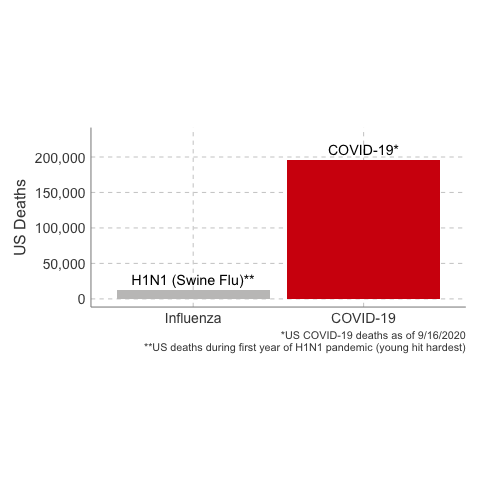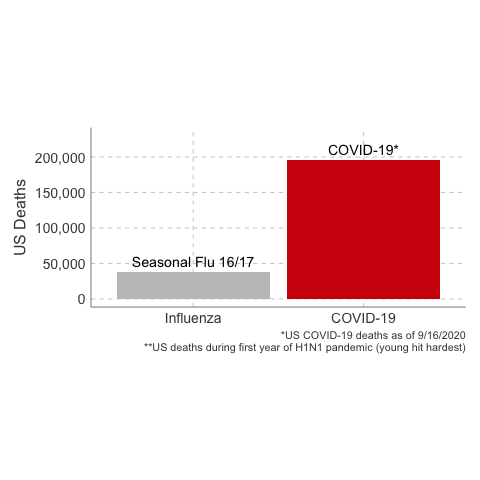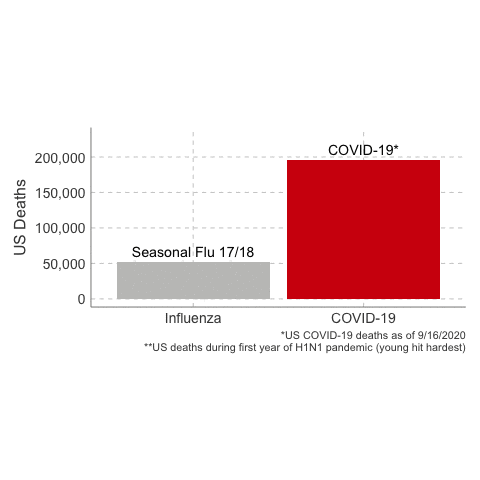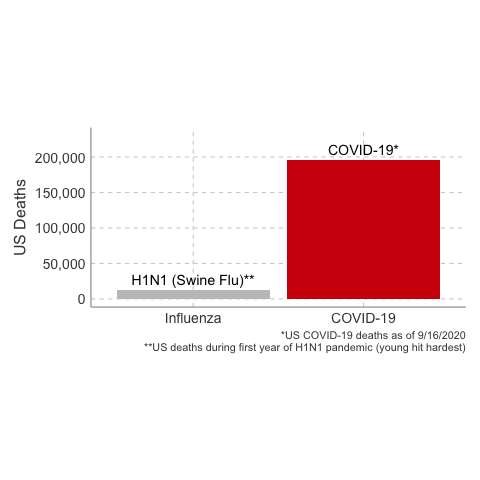
Deaths from COVID-19 vs Influenza
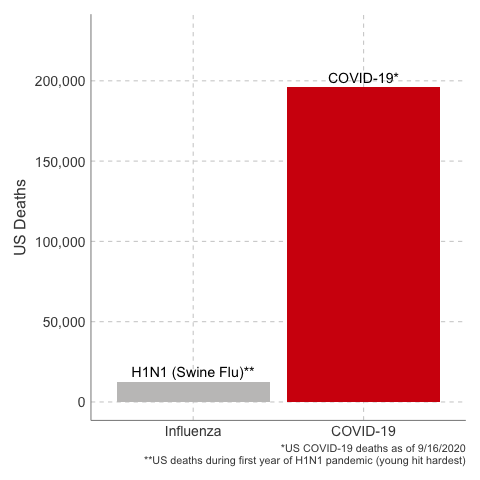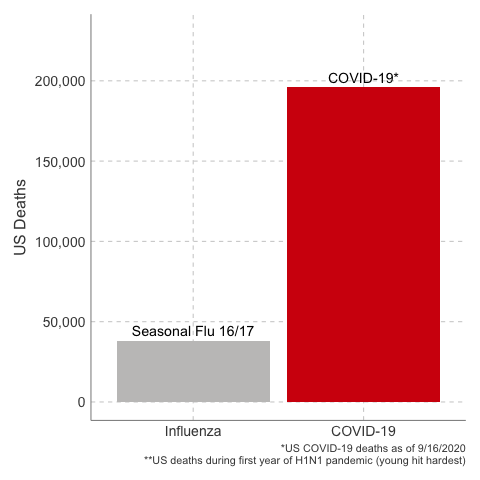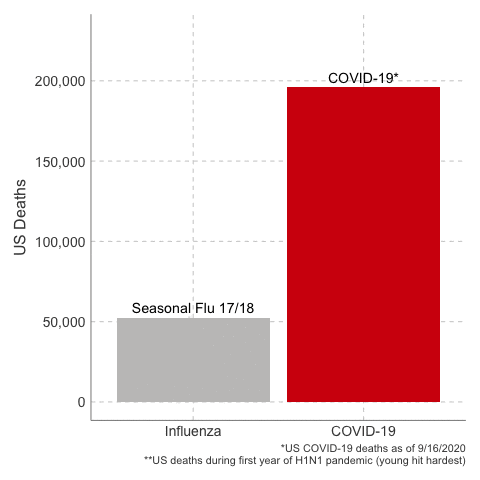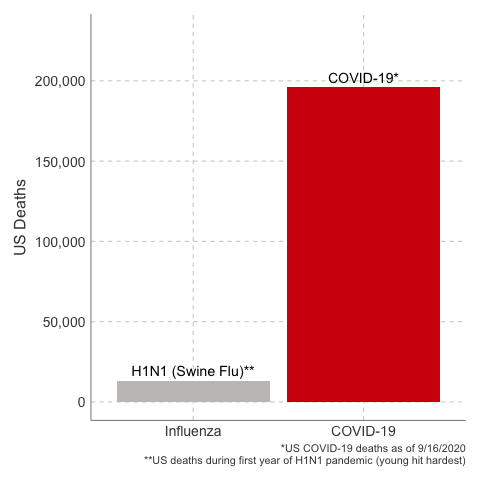
Data Sources: COVID-19, H1N1 (Swine Flu), Seasonal Flu 2016/2017, Seasonal Flu 2017/2018, Seasonal Flu 2018/2019
4. People who got moderate to severe disease from COVID-19 did not behave like people infected with other coronaviridae. This was, for all intents and purposes, a totally new disease. The complications were new and unpredictable, the best treatments and the best drugs were a question mark for the first 4 months, and the disease course was totally unfamiliar to us.
5. Drugs that worked against other coronaviridae were hypothesized (such as zinc) but were not know to work against COVID-19, so unlike influenza, we had pretty much no drugs known to be effective against COVID-19.
6. Vaccine development was started as soon as it was understood what we were dealing with, but unlike influenza, we have never made a vaccine to a coronavirus before, so we had less existing vaccine infrastructure to get us off the ground.
7. It was not predictable who would be immune to the virus, even those who had antibodies to other coronaviridae could still get the virus. Unlike H1N1, the elderly were not immune.
8. Initial data on masks was questionable (largely because we were basing our ideas on data based on influenza transmission), but over time it was found that masks and social distancing were more and more important in reducing viral spread.
My experience treating COVID patients
I’d like to tell you about my experience treating COVID-19 patients in the hospital. Now, let’s remember that anecdotes do not equate to evidence. What I was seeing may have been much better or much worse than what others were dealing with. What I say here is just the account of one senior resident physician who took care of patients on the hospital floor, and in our dedicated COVID ICU.
When COVID-19 first started being reported broadly in the press, I was in Uganda, and the cases were almost exclusively in China. By the time I made my way back to the U.S., there were increasing calls to begin social distancing. I remember, at the time, thinking that this was a large over-reaction. My only experience with the coronaviridae was when I was in medical school and I had learned that coronaviridae usually cause cold-type illnesses. It took my roommate (also a physician) making a public statement, and talking to me about the need for social distancing to get me on board. Even at that time; however, I remember social media posts abounding that ‘the flu kills more people every year’, and ‘cardiovascular deaths and cancer deaths per day are still greater than COVID deaths’, and ‘we haven’t even lost as many people as with H1N1, and they’re freaking out way more’. I even recall one of my bosses (a high level OB-Gyn) commenting that we were putting so much energy into making accommodations in the hospital for the feared influx of COVID patients, and were putting so many restrictions on activities despite the virus not yet causing as many deaths as H1N1. Physicians were not nearly uniform, initially, in their endorsement of social distancing, even though it seemed like almost everyone was worried about the PPE situation. Then, the cases started to mount. At the worst I saw it, my hospital had about 100 patients on the regular floors requiring oxygen just because of COVID-19, and about 20 people in the newly appropriated negative-pressure COVID Intensive Care Unit (ICU). At first, it was still kind of a distant experience for me though, because residents weren’t allowed to treat COVID-19 patients on the floor, and I had not been called to rotate in the COVID-ICU yet. It all changed when I joined the COVID ICU team. Now, keep in mind, I only served on that team for a week and a half. I had co-workers who were on the COVID-ICU team for an entire month, sometimes two months. Whatever experiences I had pale in comparison to what they lived.
The biggest problem with the COVID that I saw was that patients who needed hospitalization often had long stays. Some patients had been intubated and in the ICU for an entire month. I ended up feeling that one of the blessings of other diseases and pathologies was that people would ‘declare themselves’- they would often show clear signs that they were going to die soon, or that they would get better. COVID didn’t act like that. People would go the COVID ICU because they needed BIPAP or CPAP (non-intubation methods of helping people breathe), and they would get worse and need to be intubated, and then their organs would start to fail one by one. But you could never tell who would slowly get better, who was going to die despite your best efforts, and who was going to be stuck unconscious, probably uncomfortable, lonely and without any human dignity for a month at a time before they eventually died or made some minimal recovery that let them leave the ICU. We couldn’t allow visitors in the COVID ICU, so I would video conference with patient families while in my PAPR suit (basically like a HAZMAT suit but with a filtered air supply) and show them their loved ones just so they could talk to them in their drug-induced slumber. These people with bad COVID were in a completely unrecognizable form – people with wires and tubes, surrounded by machines; honestly, it was awful. We had several young people die, several people who were previously healthy leave the COVID ICU having suffered strokes from effects of the virus, or worse yet because of the therapies we were giving them, we had tens of people who had normal kidneys before who needed dialysis now, and frequent death in the elderly. To be clear – there were people who made great recoveries and left the COVID ICU a little debilitated but otherwise ok, but there were many, many more who suffered a great deal before leaving the ICU in very bad shape with new chronic health conditions from their stint with COVID.
Because of my experience, I am personally in the camp that believes that every prevented COVID-19 ICU hospitalization is a victory.
Dr. Sana Zekri, MD is a Family Medicine with Obstetrics Physician. His particular interests are in public health, global health, women’s health and working towards justice in medicine. He is currently an Assistant Clinical Professor at SUNY Upstate, in Syracuse, New York. The views expressed on this website do not necessarily reflect the official views of the author’s employers or affiliated institutions.