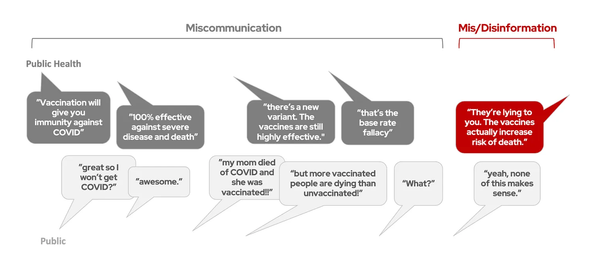
Why does it take so long to develop medical treatments?
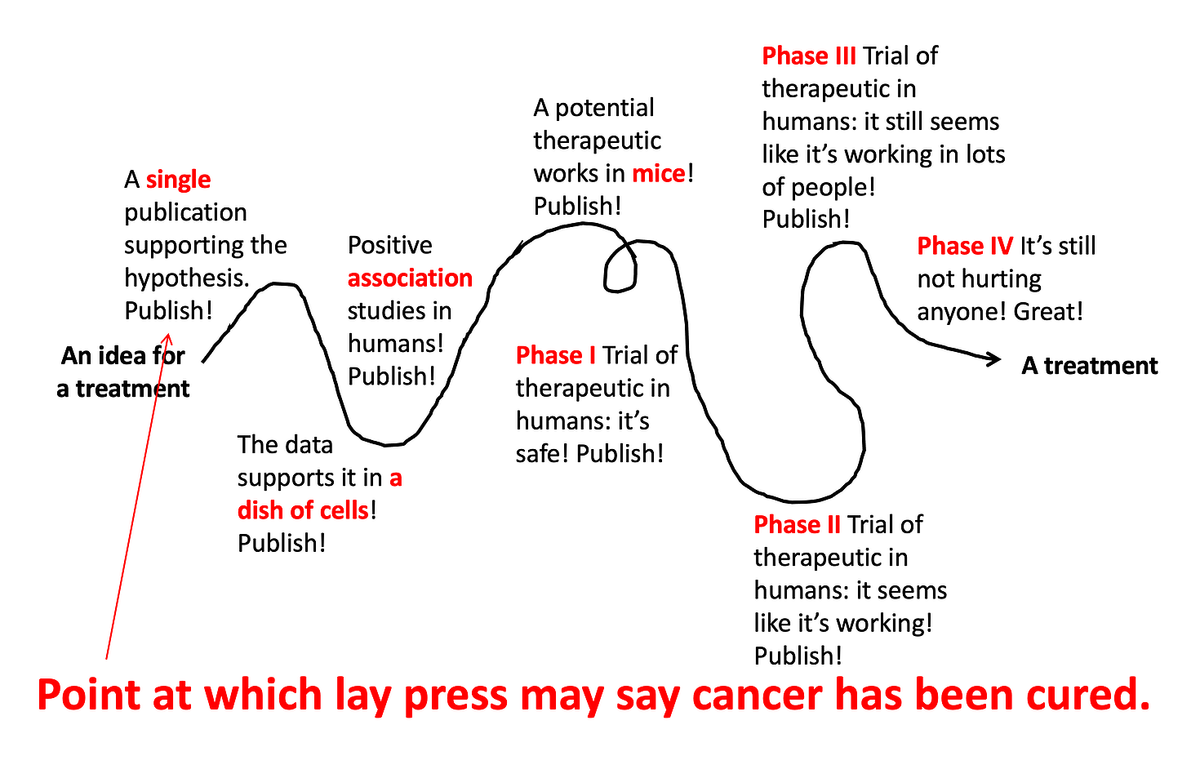
Medical research is a loooooooooong process. Like, really long. There are many different steps along the way, and from the outside it can be confusing why there was a study published years ago saying Drug X works for Disease Y, but a decade later we still don’t have a treatment. Why is this?
Medical Research is a Process
In an ideal world, developing medical treatments would look like this.
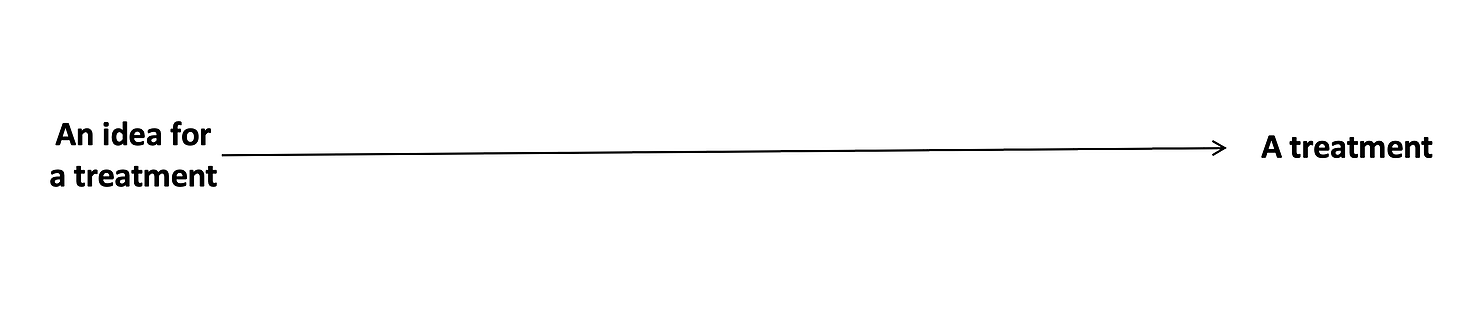
Somebody has a great idea for a new treatment, and that new treatment quickly gets tested and developed. But because biology is super complicated, and there are lots of variables that we still don’t understand well, it usually ends up looking like this:
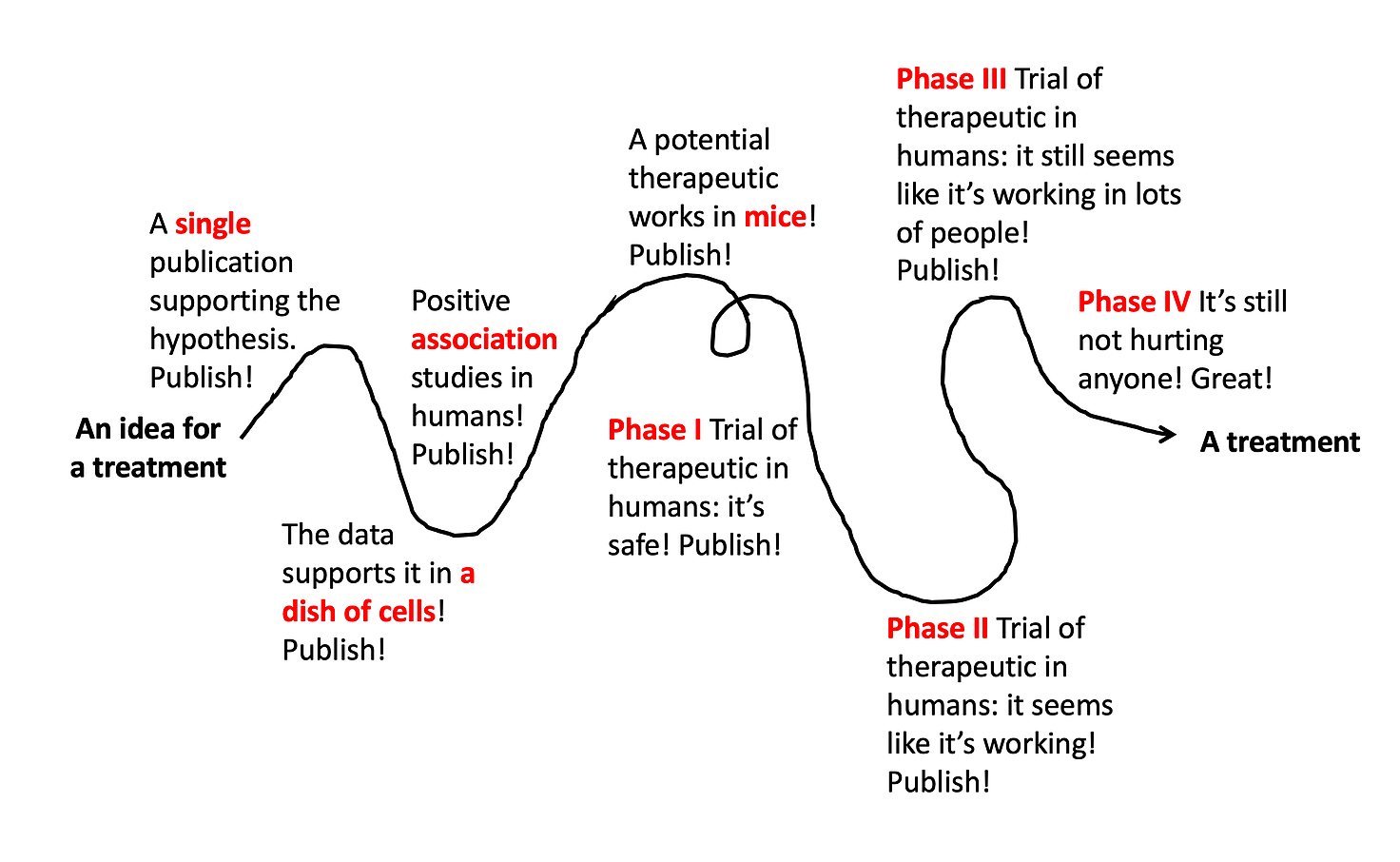
Lots of different types of studies build upon each other, ultimately leading to a well-tested, effective, and safe treatment. But even this doesn’t always work out. The vast majority of drug ideas end up failing somewhere along this path.
[Note: depending on the treatment under investigation, these steps aren’t always in exactly this order, and it’s often even messier and more squiggly path than the line drawn above. But for the sake of simplicity, I’m breaking it down into these simple steps illustrating the different types of studies that build upon each other to ultimately develop a new therapeutic.]
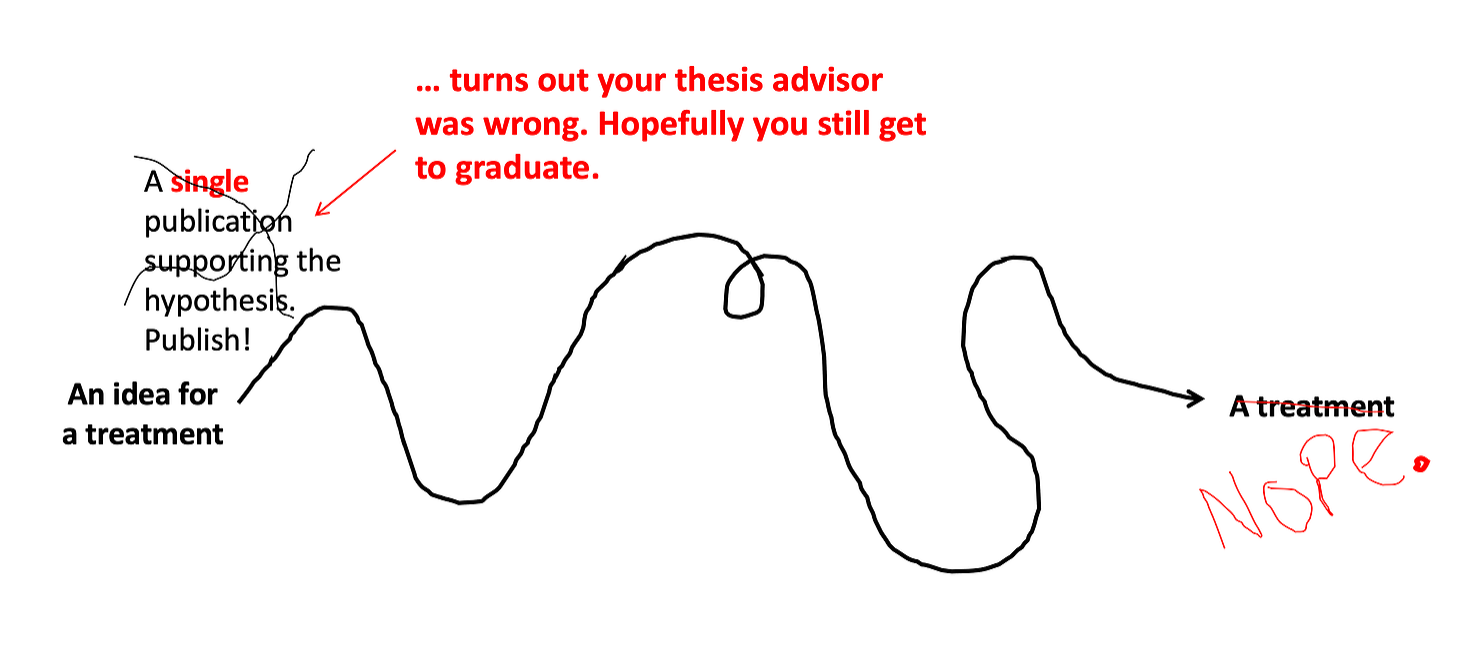
Step 1: Do the very first study.
Say a scientist or a doctor gets a great idea for a new therapeutic while drinking a beer at the pub. So they design an experiment (or in reality, more like 5-20 different experiments) and test out their idea. Depending on the type of study, the experiments that go into a single research article can take months (or more often years) to complete. Then, it’s published! The world knows! And, the PhD student who did the work gets a thesis. A success!!
But many of these ideas don’t end up working out… maybe after several months of experimenting, the data just doesn’t back it up. Some ideas sound great on paper, but fail to work right out of the gate. Even talented scientists come up with lots of ideas that don’t work.
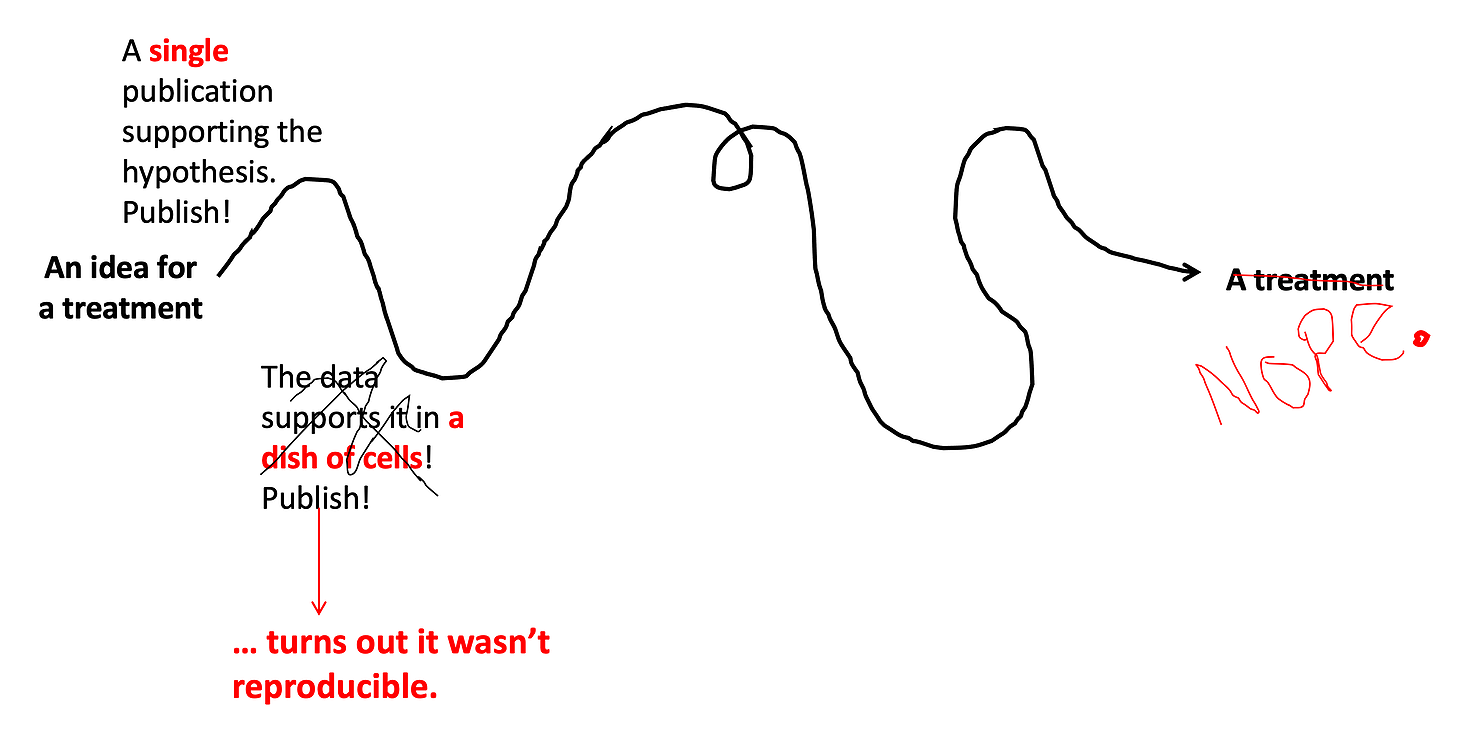
Step 2: Reproduce it.
Ok let’s say the idea worked… in a single study. It’s published! But now comes the most central tenet of scientific research: it must be reproducible. Lots of studies end up failing this test (check out this book if you’re curious why). Depending on the type of scientific question, scientists might try to reproduce the results in a cell line (cells grown in a dish). These are very common experiments and are called in vitro studies (vitro is latin for glass, so basically it translates as: in a glass tube/dish/some other glass container). In reality, these are now usually made of plastic, but that’s ok. While in vitro studies provide valuable information (and are much easier to do than trials in animals or humans), it’s important to note how early they occur on this timeline… they are considered very preliminary evidence for drug efficacy, and you can’t definitively conclude that a drug works based only on in vitro studies. (Much of the early excitement about hydroxychloroquine was based on in vitro studies, and more than one confident gent on the internet sent me these studies as evidence that “it works.” But you can see how early it is on the timeline, and why many scientists remained skeptical of the in vitro studies on hydroxychloroquine, waiting for more rigorous human trials to decide if it really works for COVID-19.)
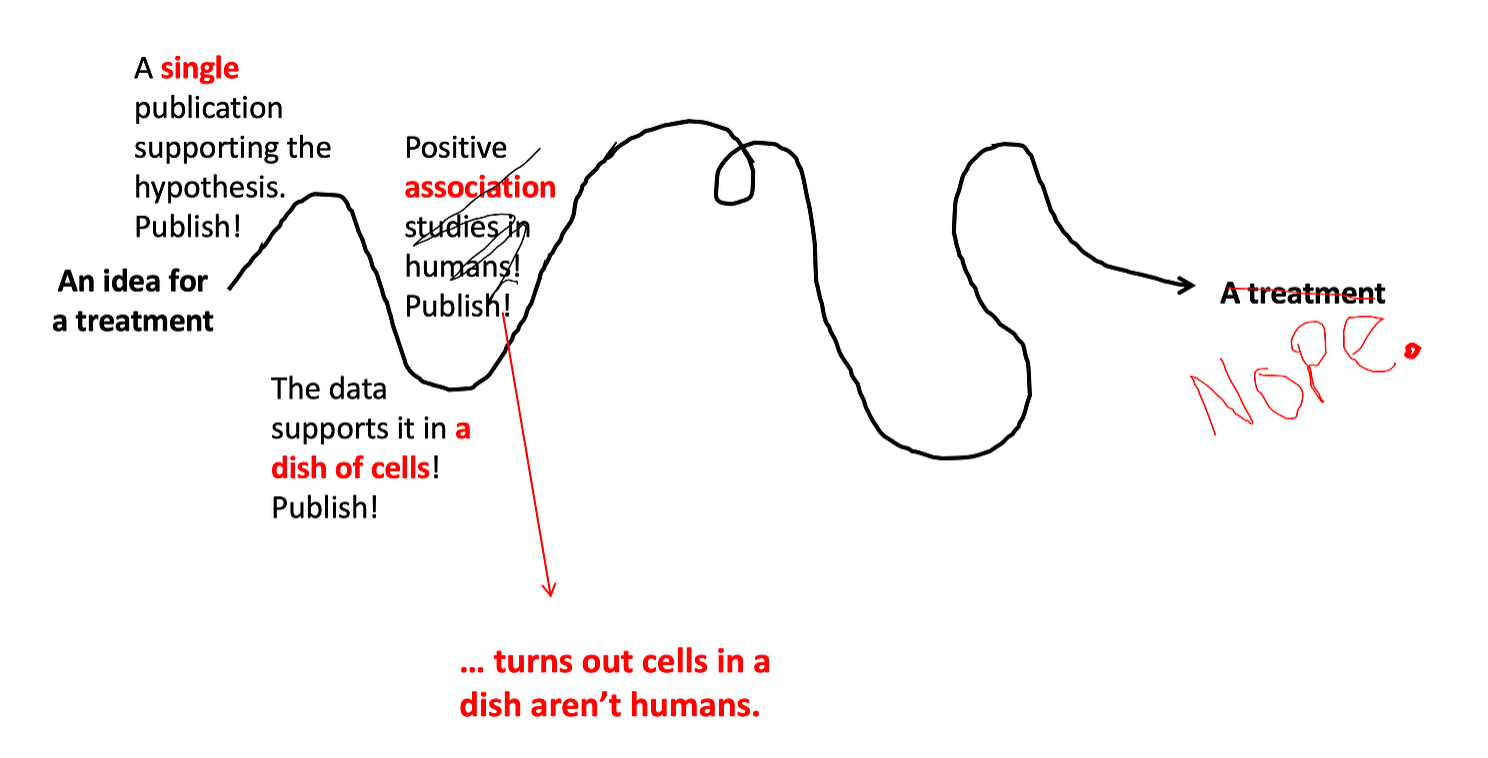
Step 3: Look at the data in humans.
The next step in the process (again depending on the therapeutic) might be to look for epidemiological data to see if the drug works. This is what happened more recently with hydroxychloroquine — because it was being prescribed as an emergency treatment, we could look back and see if the patients who got the drug did better than those who didn’t. (Usually drugs aren’t prescribed before they’ve gone all the way through this process, the pandemic was an exception). These type of observational studies provide better data than studies done in a dish of cells, but they still have problems — the main one is there is no guarantee that the people who got the drug are similar to those who didn’t. For example, maybe hydroxychloroquine was prescribed to patients who were sicker to begin with… this would confound the results of these types of studies. And studies may fail when they reach this level… just because cells in a dish are cured by the drug, that doesn’t mean that real breathing humans will be cured.
Step 4: Test it out in animals.
Most treatments will be evaluated in animals before going to human trials. Animal studies are usually one of the first major steps to test causation — is the thing you are studying really causing the effect that you think it is? Association studies in humans might fool you — some things are associated, but that doesn’t mean one is the cause of the other. In animal studies, we give them the treatment, and we can clearly tell if it’s causing the effect we expect.
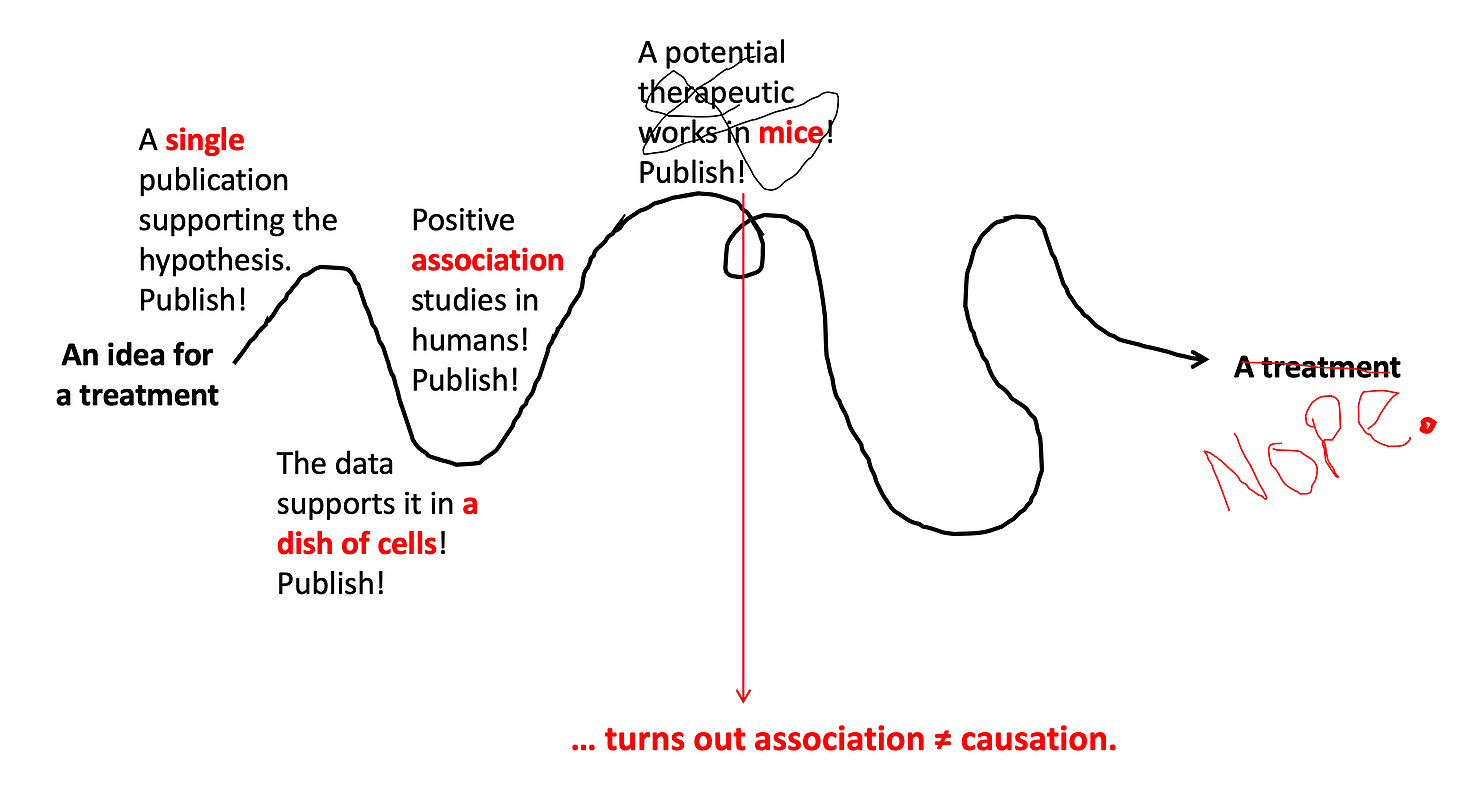
Step 5: See if it's safe in humans.
Once we have lots of in vitro experiments and animal experiments showing high promise for a drug, it’s time to test it out in humans. For new drugs that have never been used in humans before, the very first test in humans is always safety. This is called a Phase I Trial — where the primary goal is to determine if the drug is safe. These studies usually start with just a few people who are given a very low dose of the drug, and they are monitored extremely carefully for any side effects. If no side effects are detected, the dose is slowly increased until the target (treatment) dose is tested. Sometimes an extremely promising treatment will fail at this step if it has significant side effects. It does not matter how many mice the drug has cured, if people’s livers don’t tolerate it, we’re not going to use it. About one third of Phase l trials fail at this step.
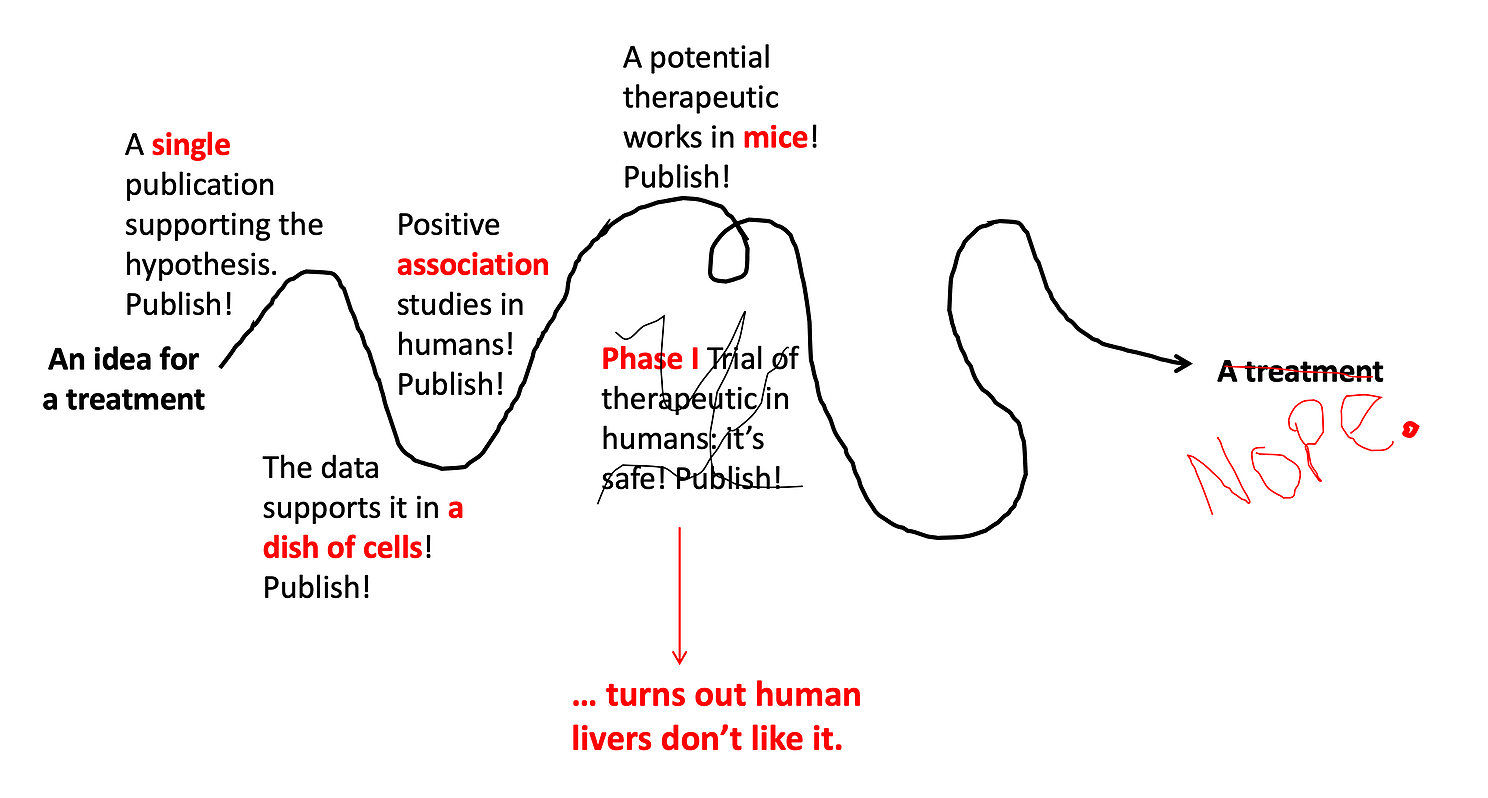
Step 6: See if it works in humans.
If a drug proves to be safe in a Phase I Trial, the next step is to see if it works in humans. Remember, just because a drug works in a dish of cells or in a bunch of mice does NOT mean it will work in human beings. Phase II Trials test this. These studies usually enroll 50-100 patients who have the disease of interest, and half are given the treatment while half are given the normal care they would get for the disease. If the people who got the treatment do better, then that is evidence the drugs works! They are also closely monitored for safety to see if there are rare side effects not detected in the Phase I Trial. About half of Phase II trials fail and don’t go onto the next step.
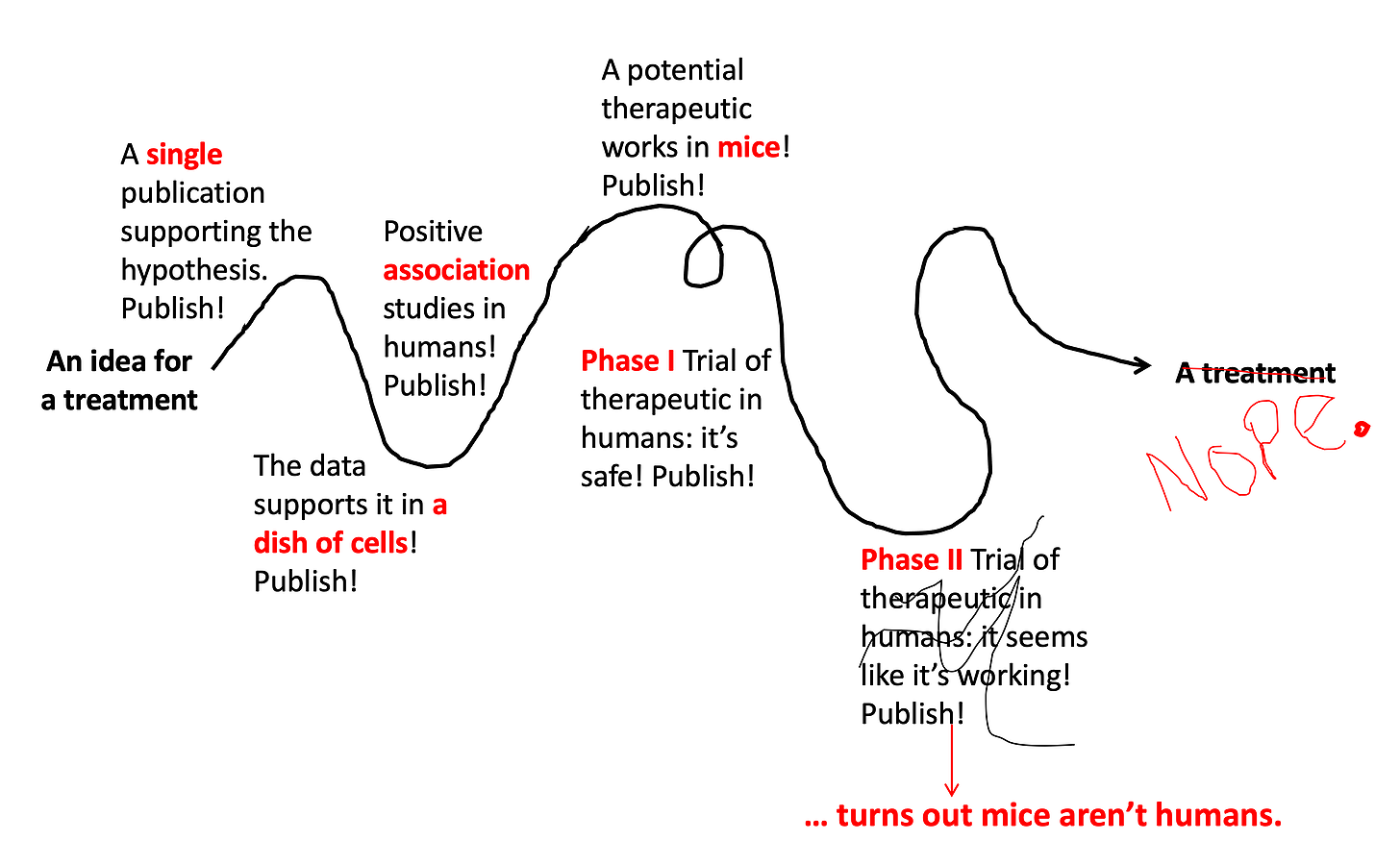
Step 7: Test it out in... more humans.
Phase III Trials test if the drug works better than the best treatment currently available. They are generally bigger than Phase II Trials (usually several hundred people) and include a placebo group to control for the placebo effect. Sadly, even though they’re so close to the finish line, even sometimes these studies end up failing (about 60% succeed). They fail for a variety of reasons — the placebo effect can be one of them.
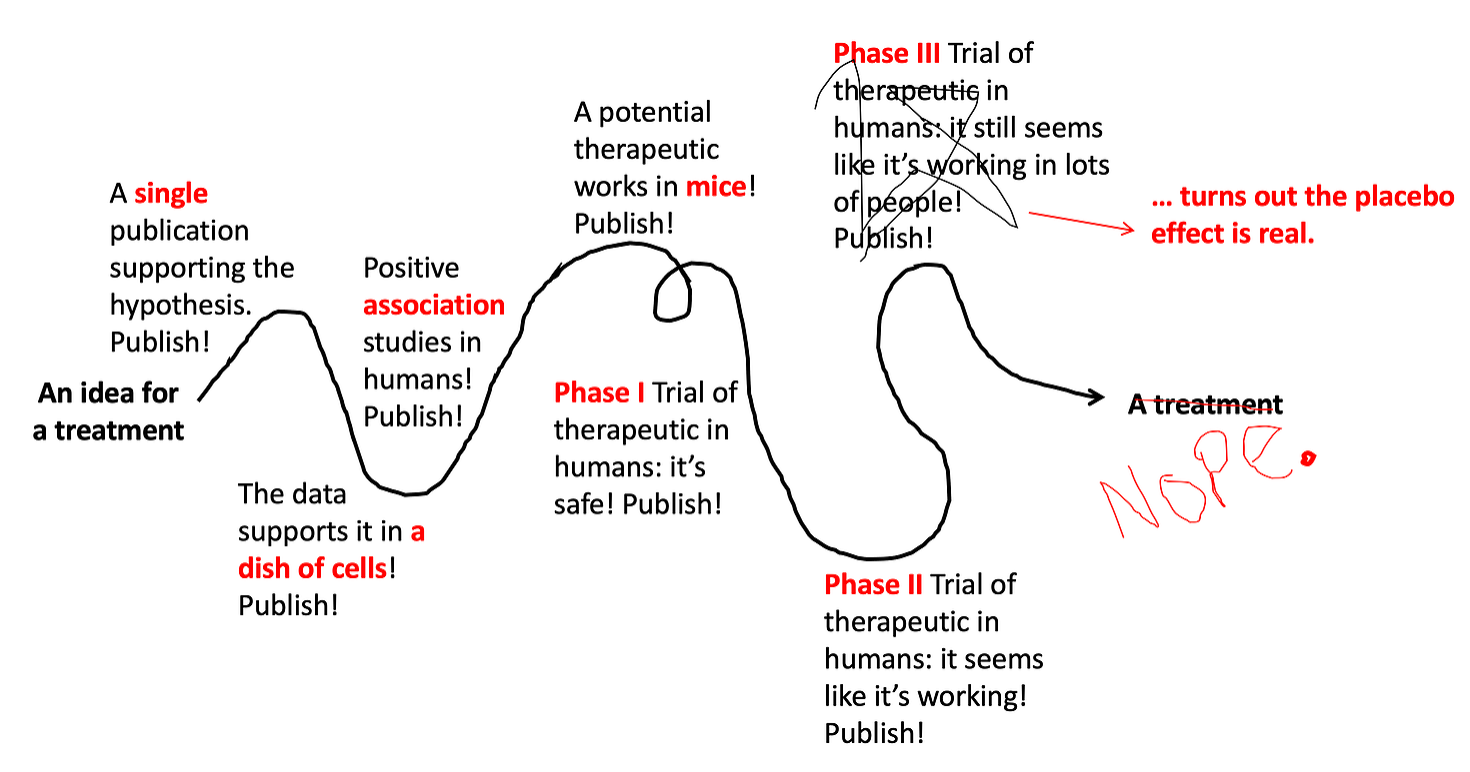
Step 8: Approve it, but keep monitoring it.
Usually once a drug has made it through a Phase III Trials it can be approved by the FDA for use. However, that isn’t the end of the story… drugs that are approved are still monitored for safety and long-term efficacy. This is sometimes called a Phase IV Trial. Maybe there was a side effect that was rare but serious, and couldn’t be detected in the earlier trials. Maybe there are severe drug interactions that weren’t detected. If there are significant side effects that prove to outweigh the benefit of the drug, the drug can be recalled even after getting FDA approval.
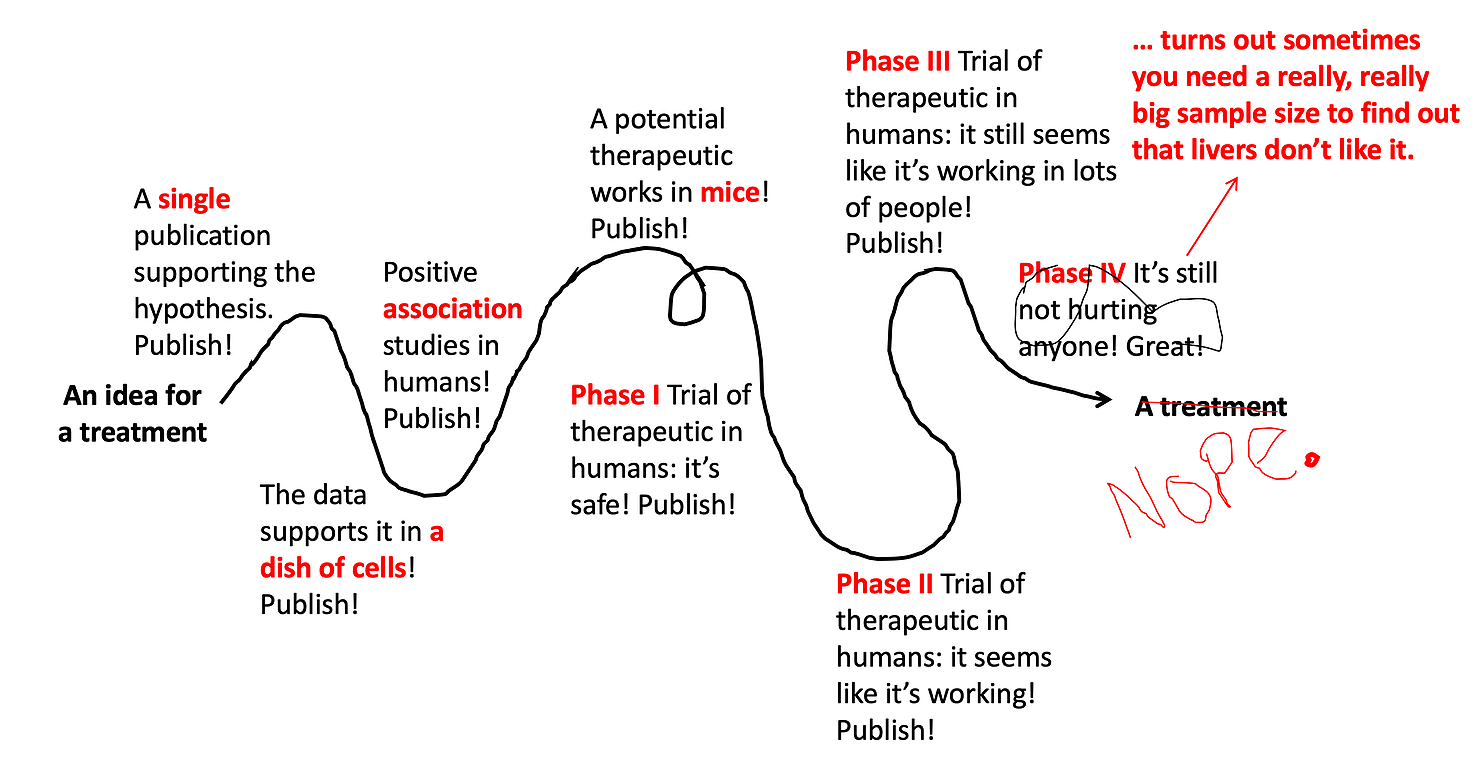
And, voila! After decades of work and millions of research dollars, your great idea has actually turned into a treatment that doctors can prescribe! You can see this is a very long process, and the vast majority of treatment ideas end up failing. However, this does not stop the press and the general public from getting excited (which is fine) and confused (which is not fine) at every step along the way.
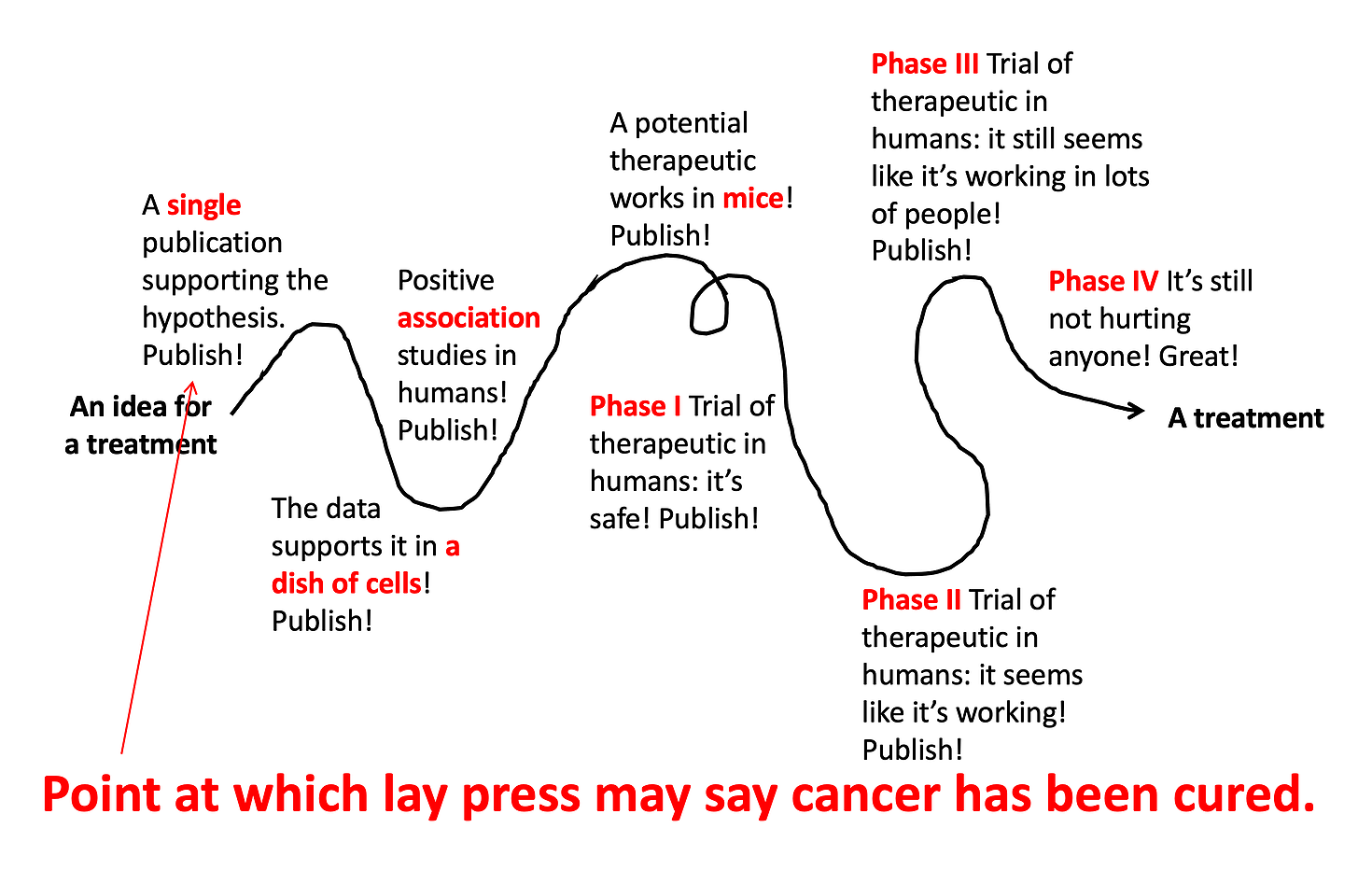
So now you know… if a study has the words in vitro, mouse, or really anything other than ‘randomized controlled trial’ in the title, it probably still has a very long way to go before we know if it’s going to work as a treatment in humans. (Check out @justsayinmice for one man’s effort to remind the world that many studies in mice are reported as if they have just saved humanity from disease.)