Racial and ethnic bias in biomedical research
Those who aren’t scientists or doctors (and maybe even some that are) may not be familiar with some of the history or present day issues around racial and ethnic bias in biomedical research. These are some of the lessons we learn in school, and I was very surprised and grieved to learn about them. There is still much to do to address these issues, but the very first step is always to become aware of the problem. So here is a start, naming a few of them:
The Tuskegee Syphilis Study
Tragically, doctors and scientists are not immune to racial bias, and some of the most egregious acts of racism have been done in the name of medical research. The Tuskegee Study of Untreated Syphilis is one of those, and is an essential lesson in understanding the roots of racial bias in the US healthcare system. This was an experiment conducted by the Public Health Service in Tuskegee, Alabama that began in 1932 and lasted 40 years, finally ending in 1972. The study enrolled 600 Black men (399 who had syphilis, and 201 who did not), and let the disease progress untreated for 40 years so they could “study the natural course of the disease.” The doctors did not tell the men they had syphilis and instead told them they were being treated for a different condition (“bad blood”). Let that sink in: the men in the study had no idea they had syphilis, and thought they were being cared for by the doctors. Furthermore, the men were actively discouraged from seeking treatment outside the study. For those of you who only have a vague inclination that syphilis is bad, let me tell you: syphilis, untreated, is horrible. It is one of the diseases that gets worse over time if not treated — the first stage is ulcers, the second stage is a body-wide rash, and the third stage is gummas (“tumors” full of the bacteria that cause syphilis, which eat away at bone and sometimes the aorta) as well as neurosyphilis, where the bacteria infect the brain and spinal cord, leading to nerve damage, blindness, and dementia. How do we treat syphilis? Simple: penicillin. It is a bacterial disease, and one of the oldest antibiotics in the book treats it. This treatment was available and standard of care for syphillis in the mid-1940s, but the doctors in this study simply decided not to use it, letting the men progress in their disease for decades and suffer instead. (References: 1 and 2)
This sounds horrible, but maybe it was just a few doctors doing something shady in secret? No, as late as 1969, the CDC reaffirmed the need for the study and gained the support of local medical societies.
What was the fallout of this “study”?
For the men who were involved in this study, the fallout was the horrible ramifications of untreated syphilis, including death. But the impact on US society was larger: once news articles condemning the study broke in 1972, trust in doctors was (understandably) broken for many in the African American community. A recent study looked at the fallout of the revelation of the Tuskegee Syphilis study, and found that “disclosure of the study in 1972 is correlated with increases in medical mistrust and mortality and decreases in both outpatient and inpatient physician interactions for older black men. Our estimates imply life expectancy at age 45 for black men fell by up to 1.5 years in response to the disclosure [of the Tuskegee Study], accounting for approximately 35% of the 1980 life expectancy gap between black and white men and 25% of the gap between black men and women.” What happens when people find out doctors are doing extremely unethical things like this study? Patients stop trusting doctors. What happens when people stop trusting doctors? They stop going to the doctor. What happens when people stop going to the doctor? They miss out on needed screening and treatments, which leads to worse health outcomes. Now before you say “but, not all doctors are like this!”… think about this for a second… if you lived in Alabama and found out that your grandfather had syphilis for 40 years and his doctors knew it but never treated him for it, and you watched your grandfather have trouble walking and get dementia and ultimately die, knowing his doctor could have prevented all of it, would you be ready to go trust a white doctor? Probably not. Now you may say, “then go to a Black doctor.” Ok… but at that point there were very few Black doctors, which is still a problem today (Black/African American doctors today make up only 5% of the US physician work-force but 12% of the US population.) This extremely unethical study is one of many examples of how the US healthcare system deeply broke trust with the African American community, tragically leading to even more health ramifications.
Did racial bias in medicine end with the Tuskegee study?
You may think to yourself “well that’s horrible, but it happened a long time ago! Things are much better now.” First, it really wasn’t that long ago — this happened in the era of my grandparents, and is still well within recent cultural memory. And second, while it’s true that the advent of ethical review boards for research studies have helped prevent episodes like this from being repeated, it’s not true that healthcare is now free from racial bias. There are many ongoing problems in direct patient care for Black/African American individuals, including lower access to health care (as assessed by various metrics), physician bias against Black patients (and other non-White patients), and even healthcare computer algorithm bias against Black patients. These along with other factors likely contribute to the fact that Black Americans have significantly worse health outcomes in many different areas, including higher all-cause mortality (age < 65), higher risk of pregnancy-associated death, and higher rate of infant mortality, to name a few. The COVID-19 pandemic has brought a fresh reminder of these health disparities: analysis of the data we have so far shows that Black patients have been disproportionately impacted by COVID-19.
Racial/ethnic bias in biomedical research studies
In addition to patient care, biomedical research still has significant racial bias problems. One of the big ones is that the vast majority of health studies done in humans predominately include White/Caucasian people. Why is this a problem? Let’s look at studies of human genetics to understand. Right now there is a huge undertaking to understand how specific gene mutations lead to disease risks. This is a very complex and rapidly developing field, but to make it very simple: scientists are trying to figure out how to use a person’s DNA sequence to predict if they are more or less at risk for certain diseases. The most well-known example is BRCA2 mutations — women who have specific mutations in the BRCA2 gene are at much higher risk for breast and ovarian cancer, so sequencing this gene and identifying people who have these mutations can lead to early interventions that prevent cancer and save lives. Everybody has a basic understanding of genes: they are the things we inherit from our mother and father, and play a big role in influencing who we are and what our bodies do. A couple things to keep in mind before we go any further: 1. your genes do not determine everything about you (not even close, and many diseases are not genetic in origin), 2. as humans, we are way more genetically similar than we are different, 3. while some gene variants are more common in certain racial/ethnic populations than others, this is no evidence of genetic inferiority/superiority/any nonsense like that. Think of these genetic variant differences as single mutations that lead to lower risk for a bad reaction to a specific drug, not anything about intelligence or ability or anything that has been proposed by the sordid history of eugenics. Ok, are we clear we’re not talking about using genetics to further racism? Good.
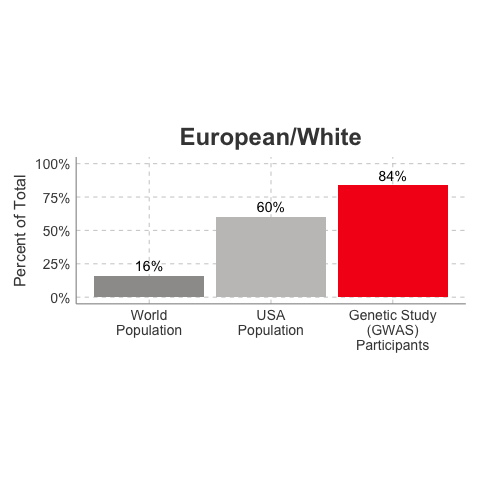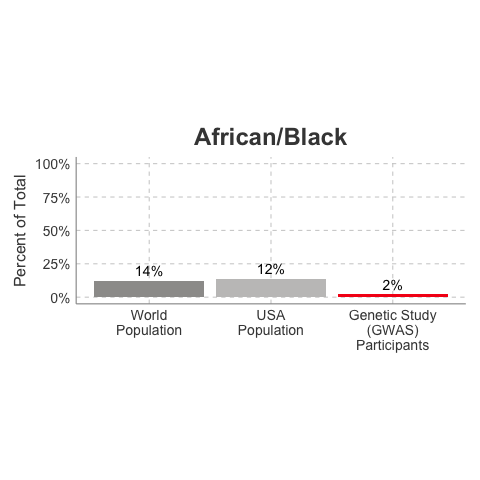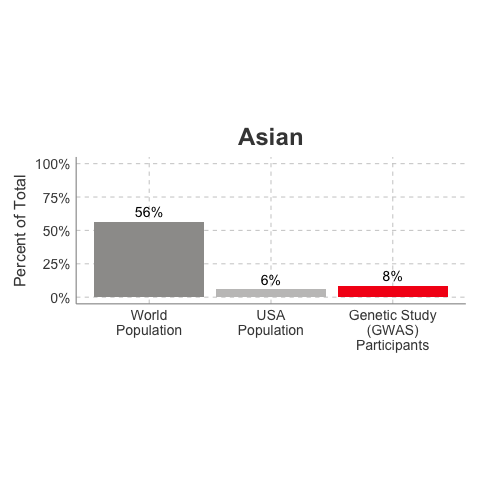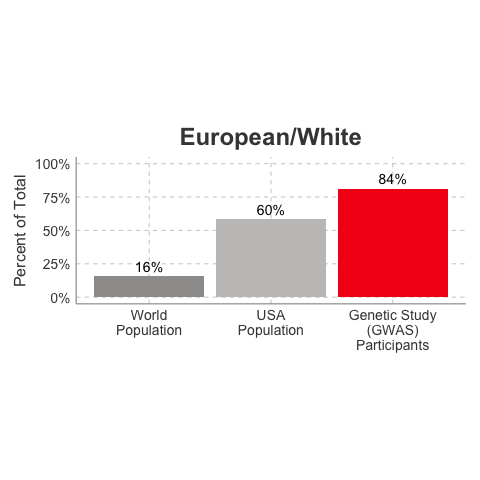
So, why is it a problem that most of our studies predominately enroll White/Caucasian individuals? Because scientists are missing data on a significant chunk of the genetic diversity in the world (see figure below). We can’t study the data we don’t have, and we’re missing data on a lot of different racial and ethnic groups. Suppose there is a genetic variant that is more common in African-descent populations than European-descent populations, and suppose that variant is associated with higher risk of heart disease. If we primarily study European-descent populations and then develop our complex algorithms to predict heart disease risk based on that data, we will likely have entirely missed a key African variant, and our algorithm will be fine-tuned towards White/Caucasian people. That’s not ok. Of course, this is a problem for other non-European races/ethnicities, not just individuals of African-descent. The National Institutes of Health knows this is a problem and encourages studies to enroll a diverse cohorts of patients, but so far this hasn’t solved the problem… we still have a long way to go, as you can see below.
Race/Ethnicity of Genome-Wide Association Study (GWAS) Participants compared to USA and World Populations
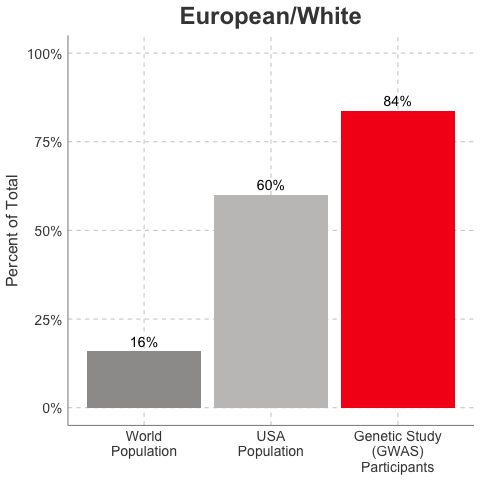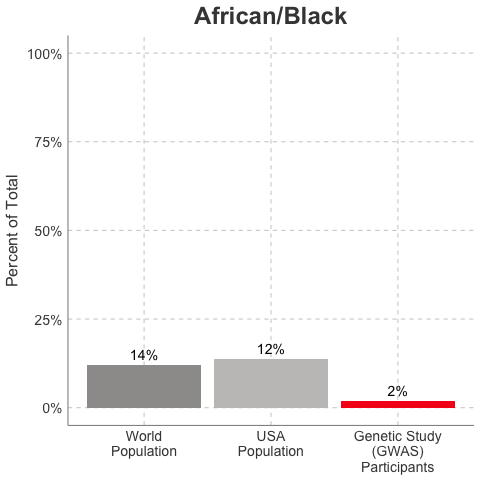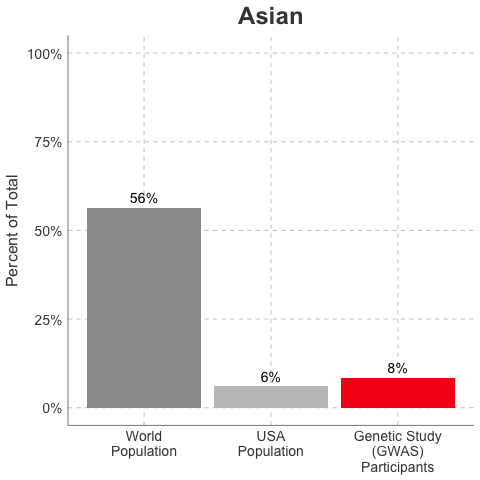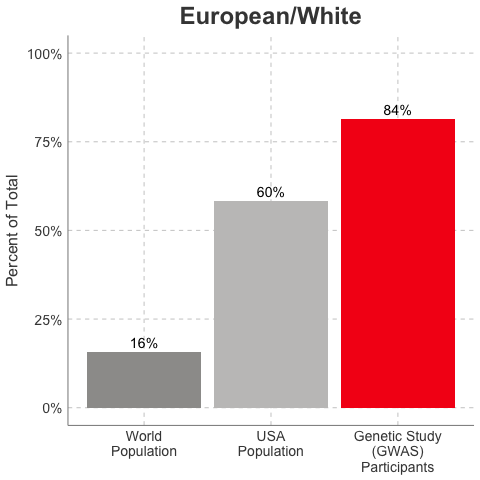
Data sources: GWAS and world population data estimated from Figure 1 of Martin et al., Nature Genetics, 2019; as I did not have access to the raw data, percentages were estimated by measuring the data in Figure 1, and may be prone to modest error. USA population data is from here.
Why is there racial bias in research studies?
So, why do these differences exist? Is it because scientists are racist and are purposefully enrolling people who are White? I guess it’s possible there’s a few examples of that happening, but that is not likely the main explanation. Studies have shown a variety of factors contribute to this discrepancy, including lack of access to the specialty care centers that often refer patients to research studies, fear of exploitation, financial limitations, and inability to take time off to participate. I propose even physical proximity to healthcare and research centers may play a factor. Enrollment for research studies often takes place at research institutions (like academic medical centers), or at hospitals associated with those institutions. I am going to use Houston, Texas as an example, as that is the city I live in. Houston contains the largest medical center in the country, the Texas Medical Center. It now encompasses over 50 hospitals and research institutions, and was first established in the 1940s. However, a decade before it was established, the location selected may have been influenced by a practice called redlining. Redlining was a system put in place in the 1930s to decide who could get housing loans after the Great Depression. Neighborhoods were graded on housing age, neighborhood land use, and most abhorrently, the race/ethnicity of the people who lived there. There were four “grades,” and people who lived in “red” neighborhoods (the lowest score, which often included neighborhoods of Black and other minorities), were denied access to loans given to other neighborhoods, effectively blocking their path to get out of poverty after the Great Depression. If you go walk these neighborhoods today, you will still see the effects of redlining: blue (“best”) neighborhoods are often full of White, wealthy individuals, and red (“hazardous”) neighborhoods are often full of non-White, poor individuals.
So where was the Texas Medical Center built? Look at the “Grades of Security” (redlining) map from the 1930s below to see. It was built near the “Best” neighborhoods.
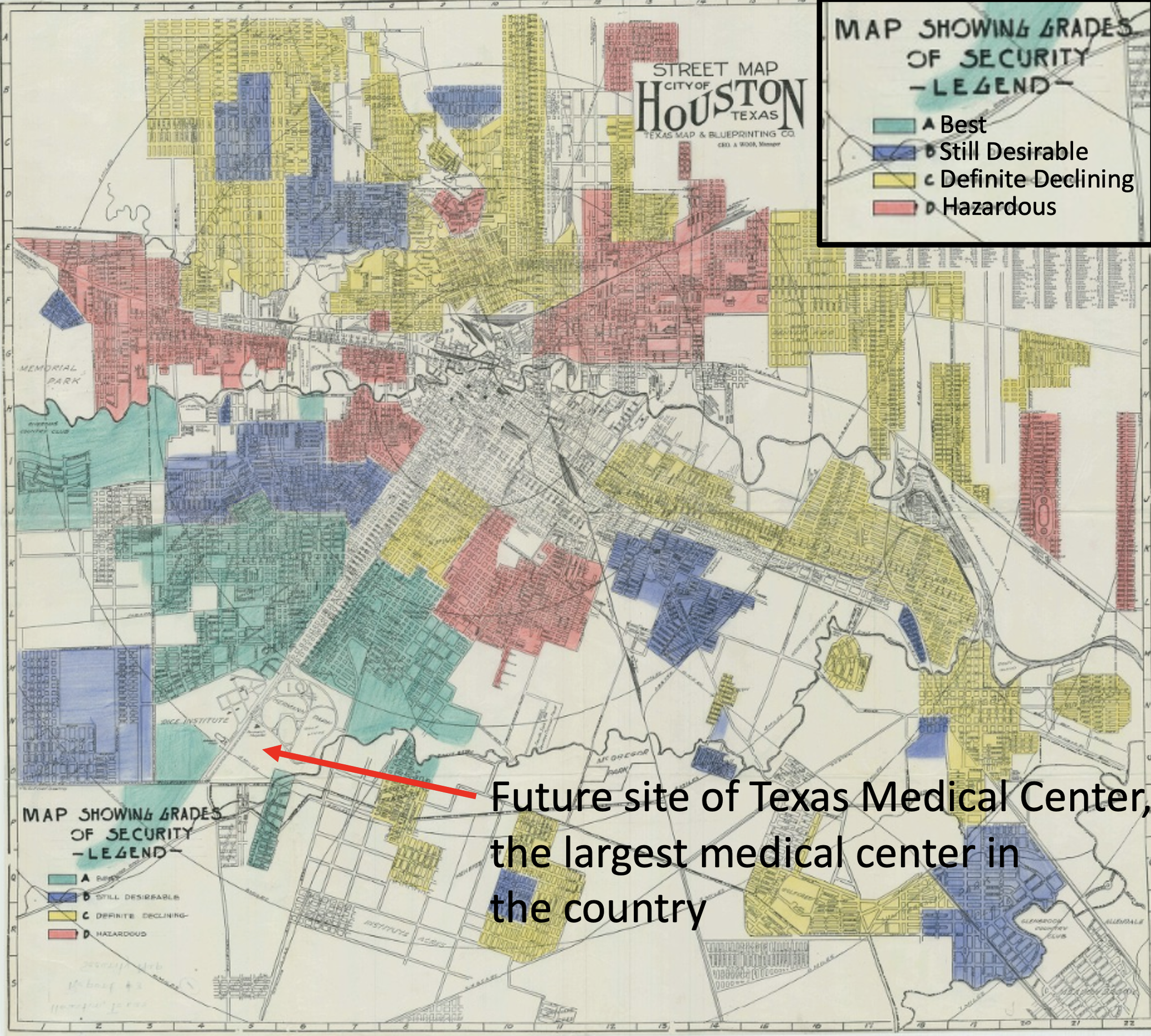
The practice of redlining has led to racial and ethnic segregation in cities that continues to this day. Check out these maps of Houston to see for yourself. To my knowledge, a systematic analysis of the location of research centers in proximity to racially and socioeconomically segregated neighborhoods has not been done (and such an analysis is certainly warranted), but it is not a stretch of the imagination to suppose that more often than not, more nice hospitals, specialty centers, and medical research centers are built in the “nice” parts of town, nor is it a stretch of the imagination to suppose that people who live further away from research institutions are less likely to travel there to participate in studies (especially for those familiar with Houston traffic). And we do know from studies that non-White individuals have more limited access to healthcare overall, for multiple complex reasons, which likely translates to more limited access to healthcare research. So, what is the solution to this racial bias in biomedical research? There are many, including improving access to healthcare for minorities (which has been improving, but there is still a ways to go), but even an effort to go recruit and enroll subjects in different neighborhoods would likely prove beneficial. This is not always easy or straightforward and may require more research personnel and outreach efforts, but it’s far from impossible.
So now you are have a little more understanding about racial/ethnic bias in healthcare and biomedical research. This is by no means an exhaustive list of issues; there are many other current-day issues in the biomedical research world that need addressing like bias in academic admissions, hiring STEM positions, and awarding of research grants. But, let this brief lesson be a start in understanding how there is still much to do to improve biomedical research and healthcare for minority groups in the United States.
A final note to any White/Caucasian individuals who may be reading this, my goal in writing this is not to shame white doctors and scientists (I am one, well, scientist/doctor-in-training). According to world-renowned shame researcher Brené Brown, shame is rarely constructive and most often destructive in the long run, and I agree: shame usually leads to defensiveness and division, not healing and reconciliation. But it’s also not ok to let fear of shame keep us from talking about real problems. So know that I am not shaming you, myself, or my colleagues, but am instead inviting y’all to be part of the solution. These are very complicated problems, and there are not easy answers, but becoming aware of the problems is a good first step.