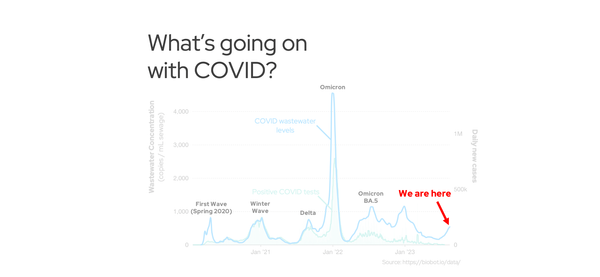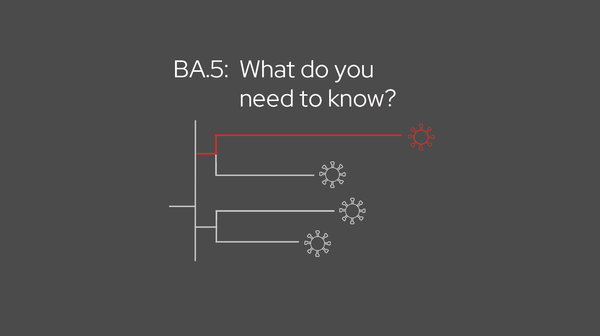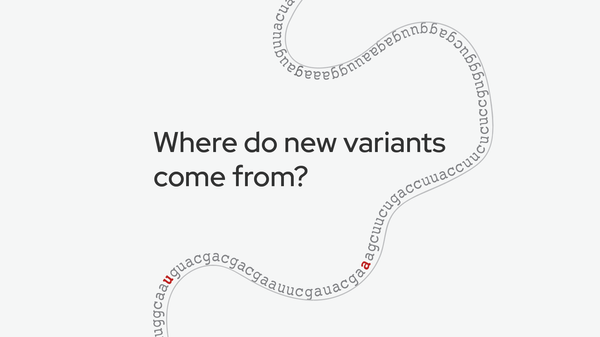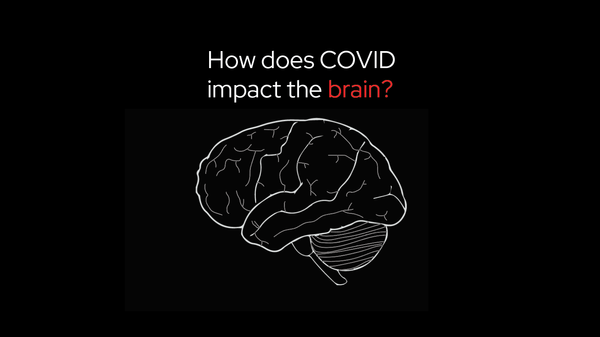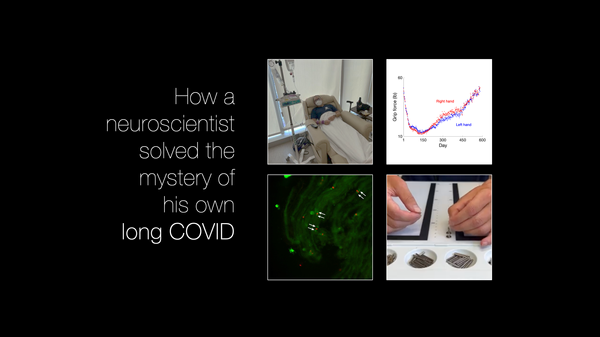
long COVID
How a neuroscientist solved the mystery of his own long COVID
When Jeff Yau started having strange symptoms after his COVID-19 infection – numbness, tingling, and shaking – he experienced what thousands of others with long COVID have found: answers were hard to find, and treatments weren’t working. But Dr. Jeff Yau was in a unique position: he’s a neuroscientist who